LOS ANGELES, June 03, 2025 (GLOBE NEWSWIRE) — Trethera Corporation (“Trethera”), a clinical stage biopharmaceutical company developing first-in-class therapies for cancer and autoimmune diseases, today announced it entered an exclusive, worldwide licensing agreement with the University of California, Los Angeles (UCLA). The license expands Trethera’s intellectual property (IP) estate for TRE-515, its lead deoxycytidine kinase (dCK) inhibitor, to include new therapeutic uses in autoimmune and inflammatory diseases.
The new license, which includes both method of use claims and compound structures, significantly extends Trethera’s patent protection in the world’s largest pharmaceutical markets. When combined with existing granted patents and filings, the new IP asset potentially secures market exclusivity for TRE-515 through at least February 2045 – supporting a long-term commercial strategy.
“This newly licensed intellectual property fortifies our already robust patent estate,” said Dr. Ken Schultz, Chairman and CEO of Trethera. “Alongside our existing composition of matter patent and ongoing proprietary discovery work, we have created a durable patent strategy to protect and maximize the full clinical and commercial value of TRE-515 across multiple therapeutic indications.”
TRE-515 is currently in Phase 1 clinical trials for cancer, an expanded clinical access program for ALS (amyotrophic lateral sclerosis, also known as Lou Gehrig’s disease), and preclinical research demonstrating promising activity in multiple autoimmune and inflammatory diseases. Trethera’s expanding IP portfolio includes multiple granted U.S. and international patents, and pending applications covering both composition and method of use claims across oncology and immunology.
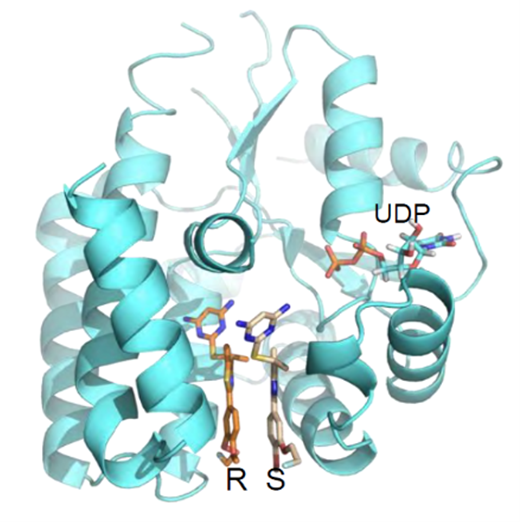
Figure 1. Co-crystal 3D structure of the drug bound to the target enzyme, dCK, at the deoxycytidine binding site.
“We are excited to partner with Trethera in the continued strategic development of intellectual property,” said Amir Naiberg, Associate Vice Chancellor and CEO & President of UCLA’s Technology Development Group. “Trethera’s deep expertise in product development, combined with UCLA’s cutting-edge research, paves the way for commercially viable treatments addressing some of the largest unmet needs in healthcare markets today.”
Dr. Peter M. Clark, UCLA professor and inventor of the licensed patent, added, “This agreement marks another key step in bringing dCK inhibitors to commercial approval. The patent claims cover both novel chemical structures as well as uses in immune-mediated disorders. Trethera’s leadership in advancing these discoveries is vital to translating our science into real-world therapies.”
Earlier, the United States Patent and Trademark Office (USPTO) issued a Notice of Allowance for a composition of matter patent covering TRE-515 through November 2041, already providing Trethera with a formidable patent runway. Global patent efforts are actively underway, with filings in major pharmaceutical markets including Europe, China, and Japan. With this newly licensed patent and a growing international IP portfolio, Trethera holds a strong patent position as it advances TRE-515 toward regulatory approval and commercialization.
About Trethera
Trethera is a clinical stage, privately held, biopharmaceutical company dedicated to pioneering the development of novel treatments for autoimmune diseases and cancers. Founded by prominent UCLA scientists, Trethera is led by experienced management and board members. Trethera’s innovative approach to targeting nucleotide metabolism led to the development of TRE-515, an orally administered capsule twice designated by the FDA as an Orphan Drug. TRE-515 is a first-in-class clinical stage drug that inhibits deoxycytidine kinase (dCK), the rate-limiting enzyme in the nucleoside salvage pathway, one of two biosynthetic pathways that generate DNA precursors. It is believed that some forms of cancer may be preferentially dependent on the salvage pathway to support tumor growth, and certain autoimmune diseases might also respond to TRE-515 treatment. Trethera is developing TRE-515 for use as a monotherapy or in combination to precisely target a metabolic vulnerability of cancer or autoimmune diseases that will transform outcomes for patients.
For more information, please visit us at trethera.com or e-mail Investor Relations at [email protected]. You can also follow Trethera on Facebook and LinkedIn.
Note on Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that Trethera believes or anticipates will or may occur in the future are “forward-looking statements,” which may often, but not always, be identified by the use of such words as “may,” “might,” “will,” “will likely result,” “would,” “should,” “estimate,” “plan,” “project,” “forecast,” “intend,” “expect,” “anticipate,” “believe,” “seek,” “continue,” “target” or the negative of such terms or other similar expressions. Although Trethera has a reasonable basis for the forward-looking statements contained herein, Trethera cautions that such statements are based on current expectations about future events and are subject to risks, uncertainties and factors relating to medical and scientific research, all of which are difficult to predict and many of which are beyond Trethera’s control, that may cause actual results to differ materially from those expressed or implied by the forward-looking statements in this press release. These potential risks and uncertainties include, without limitation: the extent to which development of any novel cancer therapies or therapies for autoimmune diseases succeeds; whether Trethera would obtain the necessary regulatory approvals to commence human trials or commercialize TRE-515 or any novel therapies resulting from such research; Trethera successfully implementing its growth strategy, including that relating to its disease therapies; the effects of the global Covid-19 pandemic; changes in economic conditions; competition; and risks and uncertainties applicable to the business of Trethera. The statements in this press release speak only as of the date hereof and Trethera does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise. The Company intends that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/6d89eb8a-6343-4fcd-89be-5ca4dbd4068e

Wall St Business News, Latest and Up-to-date Business Stories from Newsmakers of Tomorrow


