Austin, TX, USA, Aug. 21, 2025 (GLOBE NEWSWIRE) — Custom Market Insights has published a new research report titled “Biologics and Biosimilars Market Size, Trends and Insights By Product Type (Monoclonal Antibodies, Insulin, Recombinant Hormones, Fusion Proteins, Other Product Types), By Therapeutic Application (Oncology, Autoimmune Diseases, Diabetes, Blood Disorders, Other Therapeutic Applications), By End User (Hospitals, Clinics, Homecare Settings, Retail Pharmacies, Other End Users), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025 – 2034” in its research database.
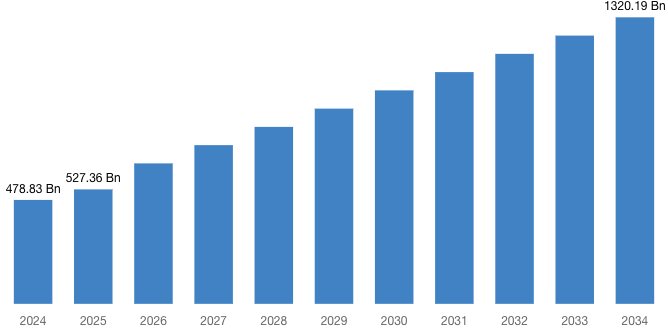
“According to the latest research study, the demand of the global Biologics and Biosimilars Market size & share was valued at approximately USD 478.83 Billion in 2024 and is expected to reach USD 527.36 Billion in 2025 and is expected to reach a value of around USD 1,320.19 Billion by 2034, at a compound annual growth rate (CAGR) of about 10.42% during the forecast period 2025 to 2034.”
Click Here to Access a Free Sample Report of the Global Biologics and Biosimilars Market @ https://www.custommarketinsights.com/request-for-free-sample/?reportid=72402
Overview
According to industry analysts at CMI, the Genetics and biosimilars markets remain buoyed by aging incidences of chronic diseases such as cancer, diabetes, and autoimmune disorders requiring long-term and targeted treatments. The newer cutting-edge biomanufacturing technologies like cell line engineering and expression systems have provided the production with more efficiency. The expiration of patent rights of several blockbuster biologics has allowed biosimilars to enter the market and commercialize themselves as relatively inexpensive alternatives.
Increasing governmental interest in healthcare cost containment, coupled with a favorable regulatory framework, has accelerated the views on biosimilars. This acceptance and adoption of biosimilars is also being encouraged by rising awareness among physicians and patients and by reimbursement policies in both developed and emerging markets.
Key Trends & Drivers
- Patent Expiry on Biologics: With the expiry of patents of important molecules, many such drugs and their costlier counterparts are entering the market. Biosimilar makers have come in with less expensive versions of in-demand therapies that include those in oncology and autoimmune diseases. This is leading to a change in competitive dynamics in the market and allowing for greater access and lower healthcare costs. Pharmaceutical companies are inclined to work on biosimilar pipelines as a means of capturing opportunities created by the expiry of patents. Accordingly, payers and governments attempt to stimulate the uptake of biosimilars to create effective treatment pathways. The patent cliff in major markets is expected to foster biosimilar launches in the next decade, especially in high-volume therapeutic areas.
- Rising Chronic Disease Burden: The increasing global demand for biologics and biosimilars is sustained by the increasing prevalence of chronic conditions such as cancer, diabetes, and rheumatoid arthritis. Biologics treat complex conditions by targeted intervention, keeping DM in long-term management, hence becoming an integral part of the treatment of today. With the aging population and changing lifestyle variables, there has been an increasing chronic disease burden worldwide. This gives an opportunity for a healthcare system to adopt innovative biologics as well as biosimilars that are cost-effective in meeting increased demand without compromising the quality of care provided. The increase in diagnoses and screening programs is further improving patient access and initiation of therapies to reinforce biologics for long-term disease control.
Request a Customized Copy of the Biologics and Biosimilars Market Report @ https://www.custommarketinsights.com/request-for-customization/?reportid=72402
- Regulatory Advancements: Product development and approval cycles have been the beneficiary of biosimilars-specific regulation in the main markets. The FDA, EMA, and PMDA have built their evaluation structures for biosimilars around science, including checks for analytical comparability, the extrapolation of indications, and interchangeability. Such advances in regulation have lowered the barriers to entry into the marketplace and made it more predictable for the manufacturers. As a result, an increased number of companies are investing in biosimilars portfolios. At the same time, regional regulatory harmonization efforts are improving market access in the regions of Asia and Latin America. With efficient approvals and increasing regulatory certainty, biosimilars are being launched faster, with acceptance building among physicians and patients all over the world.
- Innovation vs. Cost Pressure: The biologics market is shaped by an uneasy relationship between high-tech therapies and ever-escalating cost pressures. On one hand, pharmaceutical companies invest in new and advanced biologic drugs such as monoclonals for unmet clinical needs or gene therapies. On the other hand, payers and governments exert maximum cost pressure through the uptake of various biosimilars. These conflicting forces create very interesting dynamics in the market, with innovators attempting to maintain exclusivity via litigation and life-cycle management and biosimilar companies competing on price and access. This interplay will continue into strategies on pricing and shifts in market share, while investment decisions will be influenced by this process in global healthcare systems.
- Varying Adoption Across Regions: Europe leads the list of integrating biosimilars, as its early regulatory perspective and tender-based pricing supported it. In contrast, the U.S. has seen slow uptake, largely due to barriers associated with interchangeability and payer rebate agreements. Emerging markets such as India, Brazil, and South Korea are undergoing rapid development fueled by initiatives to ensure affordability and by local production capacities. Such regional variations determine strategies for market entry, pricing models, and competitive positioning. Customized education, policy alignment, and engagement with stakeholders would still be pivotal for the harmonization of the adoption trend worldwide.
- Mutating Prescriber and Patient Perception: Prescriber and patient confidence is paramount for an expanding market for biosimilars. To begin with, uptake was discouraged by concerns of efficacy, safety, and switching. But now, with real-world evidence being accumulated alongside post-marketing surveillance and clinical guidelines supporting the trust, physician education programs encourage substitution and switching, alongside payment incentives acting similarly. Patient advocacy and outreach have also provided a backdrop for building familiarity with biosimilars, especially in the treatment of chronic conditions where continuous care is required. As perceptions change, resistance at the market level decreases, thereby easing biosimilars into protocol treatments. Continuous engagement and transparent communication will therefore always be necessary to diminish behavioral and clinical inertia.
Report Scope
| Feature of the Report | Details |
| Market Size in 2025 | USD 527.36 Billion |
| Projected Market Size in 2034 | USD 1,320.19 Billion |
| Market Size in 2024 | USD 478.83 Billion |
| CAGR Growth Rate | 10.42% CAGR |
| Base Year | 2024 |
| Forecast Period | 2025-2034 |
| Key Segment | By Product Type, Therapeutic Application, End User and Region |
| Report Coverage | Revenue Estimation and Forecast, Company Profile, Competitive Landscape, Growth Factors and Recent Trends |
| Regional Scope | North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America |
| Buying Options | Request tailored purchasing options to fulfil your requirements for research. |
(A free sample of the Biologics and Biosimilars report is available upon request; please contact us for more information.)
Our Free Sample Report Consists of the following:
- The updated report for 2024 includes an introduction, an overview, and an in-depth industry analysis.
- We have included the COVID-19 Pandemic Outbreak Impact Analysis in the package.
- About 220+ Pages Research Report (Including Recent Research)
- Provide detailed chapter-by-chapter guidance on the Request.
- Updated Regional Analysis with a Graphical Representation of Size, Share, and Trends for the Year 2025
- Includes Tables and figures have been updated.
- The most recent version of the report includes the Top Market Players, their Business Strategies, Sales Volume, and Revenue Analysis
- Custom Market Insights (CMI) research methodology
(Please note that the sample of the Biologics and Biosimilars report has been modified to include the COVID-19 impact study prior to delivery.)
Request a Customized Copy of the Biologics and Biosimilars Market Report @ https://www.custommarketinsights.com/report/biologics-and-biosimilars-market/
SWOT Analysis
- Strengths: The market benefits from the strong clinical efficacy and targeted treatment for chronic and complex diseases, such as cancer, autoimmune diseases, and diabetes. Biologics represent today the standard of treatment in many high-value therapeutic areas. The biosimilars are cheaper alternatives that increase access to health care and reduce costs. Increased regulatory support, patent expiration, and an increase in real-world evidence will encourage a further increase in the uptake of biosimilars. Global pharma players pledge to consistently invest in R&D and continuously enhance their manufacturing processes. Developed and emerging markets firmly push demand, alongside advances in diagnostics, growing aging populations, and increasing health insurance coverage, thus ensuring growth over the long term.
- Weaknesses: The market-entry barrier due to high manufacturing complexity and a cost-intensive development process mainly affects smaller entities, such as biosimilars. Although the production risk for biologic products is higher owing to the need for specific infrastructure, cold-chain logistics, and intense quality control, such regulations for biosimilar approval, including analytical comparability and clinical validation, also increase the time-to-market and cost. Physician knowledge and form-filler skepticism toward biosimilars-greatly impeding rapid uptake-are becoming the biggest issues in areas where biosimilars have low penetration. Interchangeability issues, complex payer structures, and reimbursement inconsistencies across regions create inefficiencies for the markets. Furthermore, depending on only a few therapeutic areas creates further limitations to diversification and increases competitive and pricing pressures.
- Opportunities: The expiry of blockbuster biologics creates profitable opportunities for biosimilar developers to enter the high-demand market with more cost-effective alternatives. Emerging countries represent high-growth potential zones due to rising healthcare demands, favorable government policies, and burgeoning local production capabilities. Bioprocessing, formulation, and delivery technologies keep evolving to make biosimilars easier to scale up and more patient-friendly. There is a wide array of new therapeutic applications of biosimilars, including ophthalmology, endocrinology, and rare diseases, which are a big source of revenue. Value-based approaches to healthcare and tender-based procurement systems put a price advantage on biosimilars. Furthermore, further scaling via contract manufacturing, joint ventures, and strategic partnerships can help companies spread to the global markets and enter faster.
- Threats: Originator companies filing patent infringement actions and litigation during the lifetime of patents prevent the entry of biosimilars and raise compliance costs. Brand loyalty, physician inertia, and limited automatic substitution policies impede biosimilars with respect to market penetration, especially in developed markets. This aggressive pricing competition among biosimilars may eat into margins and may, in turn, deter investments. Regulatory uncertainty in some regions and slow implementation of policies on model laws may limit access.
Request a Customized Copy of the Biologics and Biosimilars Market Report @ https://www.custommarketinsights.com/report/biologics-and-biosimilars-market/
Key questions answered in this report:
- What is the size of the Biologics and biosimilar market and what is its expected growth rate?
- What are the primary driving factors that push the Biologics and Biosimilars market forward?
- What are the Biologics and Biosimilars Industry’s top companies?
- What are the different categories that the Biologics and Biosimilars Market caters to?
- What will be the fastest-growing segment or region?
- In the value chain, what role do essential players play?
- What is the procedure for getting a free copy of the Biologics and Biosimilars market sample report and company profiles?
Key Offerings:
- Market Share, Size & Forecast by Revenue | 2025−2034
- Market Dynamics – Growth Drivers, Restraints, Investment Opportunities, and Leading Trends
- Market Segmentation – A detailed analysis by Types of Services, by End-User Services, and by regions
- Competitive Landscape – Top Key Vendors and Other Prominent Vendors
Buy this Premium Biologics and Biosimilars Research Report | Fast Delivery Available – [220+ Pages] @ https://www.custommarketinsights.com/report/biologics-and-biosimilars-market/
Regional Analysis
The Biologics and biosimilars (NOACs) Market is segmented by key regions and includes detailed analysis across major countries. Below is a brief overview of the market dynamics in each country:
North America: In the biologic and biosimilar market, North America offers a lead in terms of advanced healthcare infrastructures, high R&D spending, and swift regulatory approvals. With ever-evolving biosimilar guidelines developed under the U.S. FDA and the acceptance of the interchangeable status, market access has seen an improvement. Increases in demand from hospitals, payer pressures to contain costs, and the growing incidence of chronic disease form the major growth drivers. North America has a strong presence in the pharmaceutical industry and uses a lot of biologics in areas like cancer treatment, immune system disorders, and hormone-related issues, with biosimilars becoming more common
- United States: As the leading market for biologics consumption and biosimilar launches, the country benefits from a mature regulatory framework and a large patient population. It is also the top consumer of biologics globally, while biosimilars have only recently begun to enter the treatment landscape and distribution channels. Although the relevant laws for interchangeability are limited, they are, however, significantly gaining payer judgments and inclusion in formularies.
- Canada: Canada has biosimilar regulation through capable structures in Health Canada, with switching mandates active in some provinces. The existence of government healthcare and pricing transparency gives further impetus to biosimilar uptake, especially for chronic disease management. Public reimbursement prioritizes the cheapest option, which contributes to the growth of the Canadian biologic and biosimilar market, including growth hormones and TNF inhibitors. Projects to improve physicians’ and patients’ education would further bring confidence to the market. Centralized procurement has indeed helped speed up biosimilar access in provinces.
Europe: The European Medicines Agency (EMA) views Europe as a pioneering biosimilars market due to its early regulatory approvals and early physician adoption. Countries such as Germany, the United Kingdom, and France provide the stewardship of structured pricing and substitution policies. Hospital tenders accelerate uptake along with incentives to switch, most markedly so in oncology and rheumatology. Harmonized regulatory pathways and long clinical data have provided pull and support from the national health system. Biosimilars have currently reached a very high degree of integration into regular care, hence placing Europe as the benchmark for adoption worldwide.
- Germany: Due to the nature of the healthcare system, Germany took a lead in the adoption of biosimilars, whereas physicians there have enormous autonomy in their prescriptions. Financial incentives and quota systems were set up to encourage biosimilar use across therapy areas. Competition among manufacturers is very fierce, and regional players enforce various mandates. Physician awareness is very high, and detailed post-marketing surveillance enhances confidence in switching, especially in monoclonal antibodies and insulin biosimilars. Germany’s experience is often cited as an example of the successful integration of biosimilars.
- UK.: A low-priced approach is being pursued in the UK, where the NHS guidelines encourage the early uptake of biosimilars through competitive tendering and inclusion in formulary. The switch programs, sponsored well by NHS England and the regional trusts, enable rapid biosimilar penetration. Educational campaigns and clinical decision support tools have gone on to ease prescriber resistance. Oncology and immunology remain key therapeutic areas of focus. The Government of the United Kingdom views biosimilars as strategic instruments in saving funds that could be reinvested in the broader delivery of healthcare and capacity expansion initiatives.
- France: The biosimilar market in France is driven by hospital tenders and reference pricing mechanisms, which increase competition. The government has thus set national biosimilar penetration targets and incentivizes prescribers to meet them. France emphasizes physician-led switching, ensuring transparent sharing of data and the availability of clinical guidelines. Adoption across therapeutic areas is very high in oncology, endocrinology, and gastroenterology. After some moderate initial resistance, the French biologic and biosimilar market matured to become one of the most efficient biosimilar ecosystems in Europe.
Asia Pacific: Asia is emerging as a high-growth region for biologics and biosimilars, supported by the increasing incidence of diseases, the rise of the middle class, and the growing strength of local manufacturing powers. Japan, South Korea, and Australia stand as the producers and consumers of biosimilars, with all three countries having government measures that favor local production and affordability. International standards increasingly correlate with regulatory systems. Public healthcare initiatives and growing biotech innovation investments fuel long-distance growth. On the other hand, this region significantly stands out for its increased clinical experimental activities and partnerships aimed at global biosimilar commercialization.
- Japan: Japan has developed a specialized regulatory framework for biosimilars under the aegis of the PMDA to assure safety, quality, and efficacy. It promotes biosimilars through national-level reimbursement systems and treatment guidelines. Uptake levels remain high for insulin and oncology-related therapies. Despite this, doctors have, by and large, been resistant toward biosimilars, but the uptake is slowly progressing due to ever-increasing awareness-building programs and pricing incentives. The strong push for innovation and demographic changes in Japan’s biologic and biosimilar market is guaranteeing steady demand for original biologics and their biosimilar variants in the aging-care domain.
- South Korea: South Korea has remained a big biosimilar manufacturing and innovation hub where such big names as Celltrion and Samsung Bioepis have led the way. Regulatory efficiency, together with government ambiance and export orientation, has enabled it to supply domestic and international markets. Biosimilars in hospital settings enjoy wide acceptance domestically, specifically for oncology and autoimmune diseases. This was achieved through aggressive pricing policies and stringent quality assurance programs to instill confidence in the South Korean biologic and biosimilar markets. Thus, South Korea emerged as a continued engine of growth from biopharma R&D through worldwide partnering.
- Australia: Australia supports biosimilar adoption through regulatory incentives, prescriber education, and national health arrangements under the PBS. Switching schemes and substitution policies for TNF inhibitors and insulin analogs have gained traction. The Therapeutic Goods Administration is tasked with safety equivalency measures, though the cost-saving measures account for widespread access. Participation of stakeholders, including pharmacists and clinicians, has facilitated better uptake. The Australian biologic and biosimilar market is viewed as a mature and well-structured market with a steady growth promise for biosimilars in chronic care categories.
LAMEA: LAMEA biologic and biosimilar market is varied and emerging. While regulatory frameworks vary across different countries, efforts are underway to harmonize approval processes and promote local production. Biosimilars have emerged as a solution to address the key issues of access and affordability in biosimilars, thereby expanding therapeutic possibilities. Policy development and hospital-directed procurement have taken the lead in countries such as Brazil and the Middle East. Next decade programs will create tendering systems, donor support, and international partnerships to enhance the biosimilar presence in the region.
- Brazil: has an advanced biosimilar market in Latin America, characterized by strong government support and regulatory clarity provided by ANVISA. Public health programs and procurement mechanisms promote biosimilars for a reduction in therapy costs. Target areas include autoimmune diseases, oncology, and endocrine disorders. The Brazil biologic and biosimilar market is active for both local and multinational companies. Brazil’s structured reimbursement and formulary system supports further sustained penetration of biosimilars in public and private healthcare segments.
- Saudi Arabia: Saudi Arabia’s biologic and biosimilar market could be termed the largest in the Middle East, owing to Vision 2030 healthcare reforms. The SFDA has adopted global regulatory standards, fast-tracking biosimilar approvals. The government tends to tender through centralized procurement from public hospitals and also favors biosimilar acceptance. Focus remains on oncology, insulin, and growth hormones. Local manufacturer partnerships are also taking shape to curb import dependence. Saudi Arabia is gradually establishing itself as a regional hub for biosimilars by providing educational programs that foster prescriber confidence.
Request a Customized Copy of the Biologics and Biosimilars Market Report @ https://www.custommarketinsights.com/report/biologics-and-biosimilars-market/
(We customized your report to meet your specific research requirements. Inquire with our sales team about customizing your report.)
Still, Looking for More Information? Do OR Want Data for Inclusion in magazines, case studies, research papers, or Media?
Email Directly Here with Detail Information: [email protected]
Browse the full “Biologics and Biosimilars Market Size, Trends and Insights By Product Type (Monoclonal Antibodies, Insulin, Recombinant Hormones, Fusion Proteins, Other Product Types), By Therapeutic Application (Oncology, Autoimmune Diseases, Diabetes, Blood Disorders, Other Therapeutic Applications), By End User (Hospitals, Clinics, Homecare Settings, Retail Pharmacies, Other End Users), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025 – 2034” Report at https://www.custommarketinsights.com/report/biologics-and-biosimilars-market/
List of the prominent players in the Biologics and Biosimilars Market:
- Amgen Inc
- Pfizer Inc
- Novartis AG
- Roche Holding AG
- Biogen Inc
- Eli Lilly and Company
- Sanofi
- Merck & Co Inc
- AbbVie Inc
- Johnson & Johnson
- Samsung Bioepis
- Celltrion Inc
- Teva Pharmaceutical Industries Ltd
- Sandoz
- Fresenius Kabi
- Viatris Inc
- Reddy’s Laboratories Ltd
- Biocon Limited
- Coherus BioSciences Inc
- Boehringer Ingelheim GmbH
- Others
Click Here to Access a Free Sample Report of the Global Biologics and Biosimilars Market @ https://www.custommarketinsights.com/report/biologics-and-biosimilars-market/
Spectacular Deals
- Comprehensive coverage
- Maximum number of market tables and figures
- The subscription-based option is offered.
- Best price guarantee
- Free 35% or 60 hours of customization.
- Free post-sale service assistance.
- 25% discount on your next purchase.
- Service guarantees are available.
- Personalized market brief by author.
Browse More Related Reports:
Lenacapavir Injection Market: Lenacapavir Injection Market Size, Trends and Insights By Indication (HIV Treatment, Pre-Exposure Prophylaxis (PrEP)), By Formulation (Injectable, Oral Tablets), By Distribution Channel (Branded Medicine, Generic Medicine), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
Paracetamol Market: Paracetamol Market Size, Trends and Insights By Product Type (Tablet, Capsule, Liquid Suspension, Powder), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies), By Application Type (Headache & Fever, Muscle Cramps, Cold & Cough), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
Digital Mammography Market: Digital Mammography Market Size, Trends and Insights By Technology (2D Full Field Digital Mammography, 3D Full Field Digital Mammography (Digital Breast Tomosynthesis), Contrast Enhanced Digital Mammography), By Product Type (Digital Mammography System, Display Unit, Central Processing Units, Visualization Software), By End-user (Hospitals and Surgical Centers, Breast Care Centers, Diagnostic Centers), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
US Surgical Instrument Tracking System Market: US Surgical Instrument Tracking Market Size, Trends and Insights By Technology (Barcodes, RFID), By Application (Hardware, Software, Services), By End-use (Hospitals, Ambulatory Surgical Centers), and By Region – Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025-2034
US Personalized Medicine Market: US Personalized Medicine Market Size, Trends and Insights By Product (Personalized Medicine Diagnostics, Personalized Medicine Therapeutics, Personalized Nutrition and Wellness), By Application (Oncology, CNS, Immunology, Respiratory, Liver, Rheumatology), By Technology (Big Data Analytics, Artificial Intelligence, Bioinformatics, Gene Sequencing, Drug Discovery, Companion Diagnostics, Genetics, Liquid Biopsy, Others (Predictive Modeling, Imaging)), By End-user (Hospitals, Diagnostic Centers, Research & Academic Institutes), and By Region – Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025 – 2034
Hologic Mammography Market: Hologic Mammography Market Size, Trends and Insights By Technology (Digital Mammography (2D), Digital Breast Tomosynthesis (3D), Contrast-Enhanced Mammography, AI Enhanced Systems), By Product Type (Mammography Systems, Biopsy Systems, Workstations & Software, Accessories & Detectors), By End User (Hospitals, Diagnostic Imaging Centers, Breast Care Clinics & Specialized Oncology Centers, Academic & Research Institutes), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025 – 2034
Nuclear Cardiology Market: Nuclear Cardiology Market Size, Trends and Insights By Product Type (Radiopharmaceuticals, Technetium-99m, Thallium-201, Rubidium-82, Others, Imaging Equipment, SPECT Systems, PET Systems, Hybrid Imaging Systems (SPECT/CT, PET/CT)), By Diagnostic Procedure (Myocardial Perfusion Imaging (MPI), Gated SPECT, Cardiac PET Imaging, Multi-Gated Acquisition (MUGA) Scan), By Indication (Coronary Artery Disease (CAD), Heart Failure, Cardiomyopathy, Valvular Heart Disease, Others (e.g., Arrhythmias)), By End User (Hospitals, Cardiology Centers, Diagnostic Imaging Centers, Academic & Research Institutes), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025 – 2034
Veterinary Regenerative Medicine Market: Veterinary Regenerative Medicine Market Size, Trends and Insights By Product Type (Stem Cells, Gene Therapy, Platelet-Rich Plasma, Tissue Engineering Products, Others), By Application (Orthopedic & Musculoskeletal Disorders, Wound Healing, Dermatology, Others), By Animal Type (Companion Animals, Livestock Animals, Others), By End User (Veterinary Clinics, Veterinary Hospitals, Academic & Research Institutes, Biotech/Pharmaceutical Companies, Veterinary Rehabilitation Centers), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025 – 2034
The Biologics and Biosimilars Market is segmented as follows:
By Product Type
- Monoclonal Antibodies
- Insulin
- Recombinant Hormones
- Fusion Proteins
- Other Product Types
By Therapeutic Application
- Oncology
- Autoimmune Diseases
- Diabetes
- Blood Disorders
- Other Therapeutic Applications
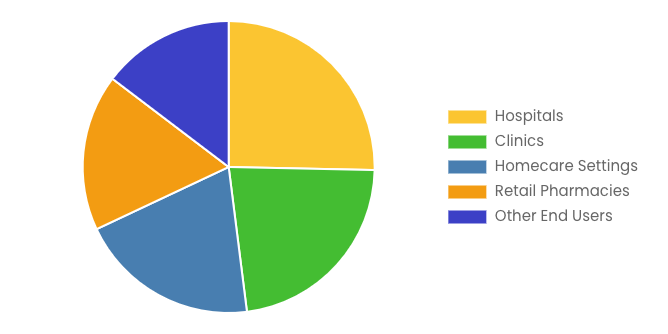
By End User
- Hospitals
- Clinics
- Homecare Settings
- Retail Pharmacies
- Other End Users
Click Here to Get a Free Sample Report of the Global Biologics and Biosimilars Market @ https://www.custommarketinsights.com/report/biologics-and-biosimilars-market/
Regional Coverage:
North America
- U.S.
- Canada
- Mexico
- Rest of North America
Europe
- Germany
- France
- U.K.
- Russia
- Italy
- Spain
- Netherlands
- Rest of Europe
Asia Pacific
- China
- Japan
- India
- New Zealand
- Australia
- South Korea
- Taiwan
- Rest of Asia Pacific
The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
Latin America
- Brazil
- Argentina
- Rest of Latin America
This Biologics and Biosimilars Market Research/Analysis Report Contains Answers to the following Questions.
- Which Trends Are Causing These Developments?
- Who Are the Global Key Players in This Biologics and Biosimilars Market? What information can you provide about their company profiles, product details, and contact information?
- What Was the Global Market Status of the Biologics and Biosimilars Market? What Was the Capacity, Production Value, Cost and PROFIT of the Biologics and Biosimilars Market?
- What Is the Current Market Status of the Biologics and Biosimilars industries? What’s the market’s competition in this industry, both company-wise and country-wise? What’s the market analysis of biologics and biosimilars, considering applications and types?
- What Are Projections of the Global Biologics and Biosimilars Industry Considering Capacity, Production and Production Value? What Will Be the Estimation of Cost and Profit? What Will Be Market Share, Supply and Consumption? What about imports and exports?
- What Is Biologics and Biosimilars Market Chain Analysis by Upstream Raw Materials and Downstream Industry?
- What Is the Economic Impact On Biologics and Biosimilars Industry? What are Global Macroeconomic Environment Analysis Results? What Are Global Macroeconomic Environment Development Trends?
- What Are Market Dynamics of Biologics and Biosimilars Market? What Are Challenges and Opportunities?
- What Should Be Entry Strategies, Countermeasures to Economic Impact, and Marketing Channels for Biologics and Biosimilars Industry?
Click Here to Access a Free Sample Report of the Global Biologics and Biosimilars Market @ https://www.custommarketinsights.com/report/biologics-and-biosimilars-market/
Reasons to Purchase Biologics and Biosimilars Market Report
- Biologics and Biosimilars Market Report provides qualitative and quantitative analysis of the market based on segmentation involving economic and non-economic factors.
- Biologics and Biosimilars Market report outlines market value (USD) data for each segment and sub-segment.
- This report indicates the region and segment expected to witness the fastest growth and dominate the market.
- Biologics and Biosimilars Market Analysis by geography highlights the consumption of the product/service in the region and indicates the factors affecting the market within each region.
- The competitive landscape incorporates the market ranking of the major players, along with new service/product launches, partnerships, business expansions, and acquisitions in the past five years of companies profiled.
- Extensive company profiles comprise a company overview, company insights, product benchmarking, and SWOT analysis for the major market players.
- The Industry’s current and future market outlook concerning recent developments (which involve growth opportunities and drivers as well as challenges and restraints of both emerging and developed regions.
- Biologics and Biosimilars Market Includes in-depth market analysis from various perspectives through Porter’s five forces analysis and provides insight into the market through Value Chain.
Reasons for the Research Report
- The study provides a thorough overview of the global Biologics and Biosimilars market. Compare your performance to that of the market as a whole.
- Aim to maintain competitiveness while innovations from established leaders drive market growth.
Buy this Premium Biologics and Biosimilars Research Report | Fast Delivery Available – [220+ Pages] @ https://www.custommarketinsights.com/report/biologics-and-biosimilars-market/
What does the report include?
- Drivers, restrictions, and opportunities are among the qualitative elements covered in the worldwide Biologics and Biosimilars market analysis.
- The competitive environment of current and potential participants in the Biologics and Biosimilars market is covered in the report, as well as those companies’ strategic product development ambitions.
- According to the component, application, and industry vertical, this study analyzes the market qualitatively and quantitatively. Additionally, the report provides comparable data for the key regions.
- We have provided actual market sizes and forecasts for each of the aforementioned segments.
Who should buy this report?
- Participants and stakeholders worldwide Biologics and Biosimilars market should find this report useful. The research will be useful to all market participants in the Biologics and Biosimilars industry.
- Managers in the Biologics and Biosimilars sector are interested in publishing up-to-date and projected data about the worldwide Biologics and Biosimilars market.
- Governmental agencies, regulatory bodies, decision-makers, and organizations want to invest in Biologics and Biosimilars products’ market trends.
- Market insights are sought for by analysts, researchers, educators, strategy managers, and government organizations to develop plans.
Request a Customized Copy of the Biologics and Biosimilars Market Report @ https://www.custommarketinsights.com/report/biologics-and-biosimilars-market/
About Custom Market Insights:
Custom Market Insights is a market research and advisory company delivering business insights and market research reports to large, small, and medium-scale enterprises. We assist clients with strategies and business policies and regularly work towards achieving sustainable growth in their respective domains.
CMI is a one-stop solution for data collection and investment advice. Our company’s expert analysis digs out essential factors that help us understand the significance and impact of market dynamics. The professional experts apply clients inside on the aspects such as strategies for future estimation fall, forecasting or opportunity to grow, and consumer survey.
Follow Us: LinkedIn | Twitter | Facebook | YouTube
Contact Us:
Joel John
CMI Consulting LLC
1333, 701 Tillery Street Unit 12,
Austin, TX, Travis, US, 78702
USA: +1 737-734-2707
India: +91 20 46022736
Email: [email protected]
Web: https://www.custommarketinsights.com/
Blog: https://www.techyounme.com/
Blog: https://businessresearchindustry.com
Blog: https://www.technowalla.com/
Blog: https://marketresearchtrade.com/
Buy this Premium Biologics and Biosimilars Research Report | Fast Delivery Available – [220+ Pages] @ https://www.custommarketinsights.com/report/biologics-and-biosimilars-market/

Wall St Business News, Latest and Up-to-date Business Stories from Newsmakers of Tomorrow