LOS ANGELES, May 21, 2025 (GLOBE NEWSWIRE) — Trethera Corporation (“Trethera”), a clinical stage biopharmaceutical company developing first-in-class therapies for cancer and autoimmune diseases, announced today the appointment of Benjamin Greenberg, MD, MHS, to its Scientific Advisory Board. Dr. Greenberg will assist in evaluating the clinical development of Trethera’s lead asset, TRE-515, in demyelinating autoimmune diseases. A demyelinating disease is any condition that causes damage to the protective covering (myelin sheath) that surrounds nerve fibers. When the myelin sheath is damaged, nerve impulses slow or even stop, causing neurological problems.
Dr. Greenberg is an internationally recognized neurologist specializing in rare autoimmune disorders of the central nervous system. He currently serves as Vice Chair of Clinical and Translational Research and Professor of Neurology and Pediatrics at UT Southwestern Medical Center. Dr. Greenberg completed his medical degree at Baylor College of Medicine, followed by an internal medicine internship at Rush–St. Luke’s Presbyterian Hospital and a neurology residency at Johns Hopkins Hospital, where he later served as co-director of the Transverse Myelitis Center. Since joining UT Southwestern in 2009, he has founded multiple pioneering programs, including the Transverse Myelitis and Neuromyelitis Optica Program and the Pediatric Demyelinating Disease Program.
“It’s fantastic to have Dr. Greenberg join our team,” said Immunology Advisory Board Chairman Dr. Peter M. Clark of UCLA. “With his addition, we now have an exceptional and purpose-driven team at Trethera with the world’s foremost experts from Harvard, Stanford, and UT Southwestern to provide scientific answers as well as enroll patients in our forthcoming neuroimmunology Phase 1 clinical trials to transform patient care.”
“I’m honored to have Dr. Greenberg join the Scientific Advisory Board and support the advancement of TRE-515 as a potential treatment for patients with rare demyelinating diseases,” said Dr. Ken Schultz, Chairman and CEO of Trethera. “Safe and effective treatments for these neurologic conditions represent a significant unmet need, particularly in pediatric populations, where existing therapies often carry substantial side effects and deliver inconsistent results.”
Dr. Greenberg’s research and clinical expertise focus on demyelinating and inflammatory disorders such as acute disseminated encephalomyelitis (ADEM), optic neuritis, neuromyelitis optica spectrum disorder (NMOSD), and transverse myelitis. As the principal investigator of major national collaborative studies, he has contributed extensively to the discovery of novel biomarkers and the development of biorepository protocols that support precision diagnostics and treatment strategies. Dr. Greenberg’s appointment to the Scientific Advisory Board further strengthens Trethera’s commitment to advancing innovative therapies for autoimmune neurologic diseases.
Trethera’s clinical stage and first-in-class drug, TRE-515, holds FDA Orphan Drug status for both optic neuritis and ADEM. FDA Orphan Drug designation confers substantial advantages, including a faster path to market approval, FDA assistance in designing clinical trials, exemption from the $4M drug approval application fee, and eligibility for seven years of marketing exclusivity. Should the FDA approve TRE-515 for commercial use in ADEM, Trethera would be eligible for a pediatric priority review voucher. TRE-515 is currently being evaluated in a Phase 1 dose escalation solid tumors clinical trial.
ADEM is an autoimmune disease, affecting 15,000 patients a year in the United States, with most cases occurring in six to eight year-old children. ADEM can present with fever and difficulty walking that progresses to loss of consciousness, coma, and even death. Optic neuritis typically occurs in adult patients, manifesting with rapid vision loss in one or both eyes, with up to one in four patients never fully recovering their eyesight. Over 100,000 cases of optic neuritis occur annually in the US and have a close association with multiple sclerosis (MS).
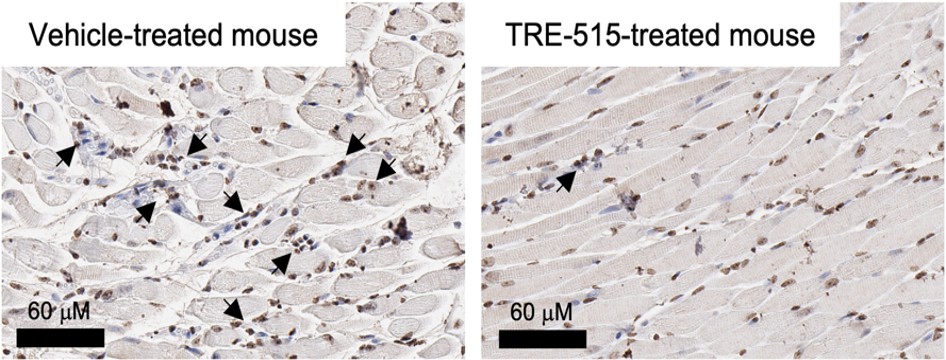
Figure 1: Representative stained sections of the optic nerve from mice experiencing optic neuritis. Arrows point to regions of leukocyte infiltration.
Sources: Cleveland Clinic 2021; Bennett 2019; Yang 2017; Wilhelm 2015
About Trethera
Trethera is a clinical stage, privately held, biopharmaceutical company dedicated to pioneering the development of novel treatments for autoimmune diseases and cancers. Founded by prominent UCLA scientists, Trethera is led by experienced management and board members. Trethera’s innovative approach to targeting nucleotide metabolism led to the development of TRE-515, an orally administered capsule twice designated by the FDA as an Orphan Drug. TRE-515 is a first-in-class clinical stage drug that inhibits deoxycytidine kinase (dCK), the rate-limiting enzyme in the nucleoside salvage pathway, one of two biosynthetic pathways that generate DNA precursors. It is believed that some forms of cancer may be preferentially dependent on the salvage pathway to support tumor growth, and certain autoimmune diseases might also respond to TRE-515 treatment. Trethera is developing TRE-515 for use as a monotherapy or in combination to precisely target a metabolic vulnerability of cancer or autoimmune diseases that will transform outcomes for patients.
For more information, please visit us at trethera.com or e-mail Investor Relations at [email protected]. You can also follow Trethera on Facebook and LinkedIn.
Note on Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that Trethera believes or anticipates will or may occur in the future are “forward-looking statements,” which may often, but not always, be identified by the use of such words as “may,” “might,” “will,” “will likely result,” “would,” “should,” “estimate,” “plan,” “project,” “forecast,” “intend,” “expect,” “anticipate,” “believe,” “seek,” “continue,” “target” or the negative of such terms or other similar expressions. Although Trethera has a reasonable basis for the forward-looking statements contained herein, Trethera cautions that such statements are based on current expectations about future events and are subject to risks, uncertainties and factors relating to medical and scientific research, all of which are difficult to predict and many of which are beyond Trethera’s control, that may cause actual results to differ materially from those expressed or implied by the forward-looking statements in this press release. These potential risks and uncertainties include, without limitation: the extent to which development of any novel cancer therapies or therapies for autoimmune diseases succeeds; whether Trethera would obtain the necessary regulatory approvals to commence human trials or commercialize TRE-515 or any novel therapies resulting from such research; Trethera successfully implementing its growth strategy, including that relating to its disease therapies; the effects of the global Covid-19 pandemic; changes in economic conditions; competition; and risks and uncertainties applicable to the business of Trethera. The statements in this press release speak only as of the date hereof and Trethera does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise. The Company intends that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/556b8598-d3fa-47a1-b884-32fa0d1981bc

Wall St Business News, Latest and Up-to-date Business Stories from Newsmakers of Tomorrow



