

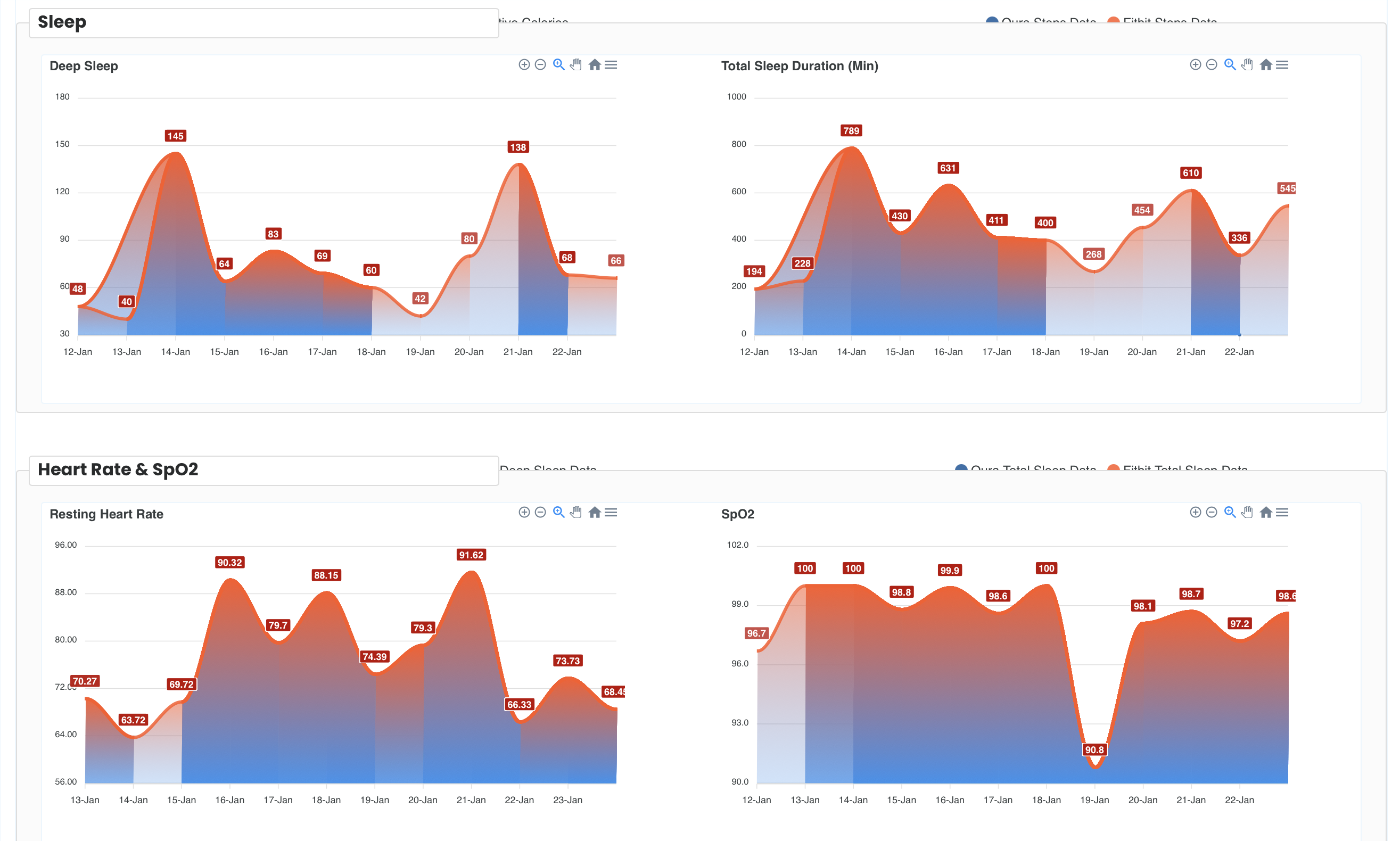
EAGAN, Minn., May 15, 2025 (GLOBE NEWSWIRE) — ABM Respiratory Care, an innovator in respiratory therapy solutions, announced today the initiation of a multi-center home care study in collaboration with Delve Health, a trailblazer in decentralized clinical trial technology, to evaluate the impact of its BiWaze® Clear System in patients living with bronchiectasis. The study will assess how the BiWaze Clear System affects respiratory health over a six-month period.
The FDA-cleared BiWaze Clear System delivers a combination of lung expansion, high frequency oscillation, and aerosol therapy designed to help patients mobilize and clear airway secretions. This post-market study will enroll approximately 85 patients across the United States, ages 5 to 85, replacing their existing airway clearance therapy with the BiWaze Clear System.
“Our goal is to demonstrate that BiWaze Clear can significantly reduce pulmonary exacerbations in bronchiectasis patients and improve their quality of life when used in the home setting,” said Leah Noaeill, Vice President of Marketing and Clinical Affairs at ABM Respiratory Care. “We’re excited to partner with Delve Health to capture meaningful data through remote monitoring, making it easier for patients and physicians to track therapy effectiveness in real time.”
Key Study Highlights:
- Next-Generation Respiratory Care: The BiWaze® Clear System represents a new approach to airway clearance in the home care environment. This trial will validate its potential to transform treatment paradigms.
- Patient-Centric Innovation: Delve Health’s decentralized trial platform enables patients to participate remotely, reducing the burden of traditional clinical trials while ensuring a diverse and inclusive participant pool. Wearable integration and telemedicine capabilities will provide real-world data in real time.
- Supporting CMS Priorities: The trial aligns with CMS’s focus on value-based care and remote patient monitoring, demonstrating how innovative technologies like the BiWaze Clear can improve patient outcomes, reduce hospital readmissions, and lower healthcare costs.
- Accelerating Impact: By combining ABMRC’s airway clearance system with Delve Health’s agile trial infrastructure, the partnership aims to deliver actionable insights faster, bringing life-changing respiratory solutions to market sooner.
The study will also collect safety data throughout the six-month therapy period, with oversight and adverse event reporting managed in accordance with FDA and ICH-GCP guidelines.
Wessam Sonbol, CEO of Delve Health, added, “At Delve Health, we believe the future of clinical trials is decentralized, digital, and patient-first. Partnering with ABM Respiratory Care on this trial allows us to bring that vision to life while supporting the development of a device that has the potential to revolutionize respiratory care and align with CMS’s goals for improved patient outcomes and cost efficiency.”
Patient enrollment will begin in May 2025, and the study is expected to run for 18 months.
About ABM Respiratory Care
Founded in 2017, ABM Respiratory Care is dedicated to advancing patient care by developing intelligent, clinically differentiated, and innovative respiratory care solutions to help people breathe better inside and outside the hospital. Our connected platform is designed to improve respiratory therapy by providing deeper breathing, improved oxygen exchange, reduce aerosol emission exposure, and communication for better disease management for people with compromised respiratory systems, in any care setting around the world. For more information visit, www.abmrc.com.
Investor and Media Contact:
Leah Noaeill
VP of Marketing and Clinical Affairs, ABM Respiratory Care
[email protected]
1.877.226.7201
About Delve Health:
Delve Health is revolutionizing clinical trials through its decentralized, digital-first platform. By integrating wearable technology, telemedicine, and advanced analytics, Delve Health empowers researchers to conduct trials that are faster, more inclusive, and patient-centric. For more information visit, www.delvehealth.com.
Media Contact:
Wessam Sonbol
[email protected]
952.200.6228
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/e14d71d9-8136-4c41-bba6-511ffc5967ff
https://www.globenewswire.com/NewsRoom/AttachmentNg/1c83f036-7a98-4063-b29e-aadb7ec17e80
https://www.globenewswire.com/NewsRoom/AttachmentNg/f5e4288b-db9f-433f-bae6-2ea0418316dd

Wall St Business News, Latest and Up-to-date Business Stories from Newsmakers of Tomorrow


